Our Product
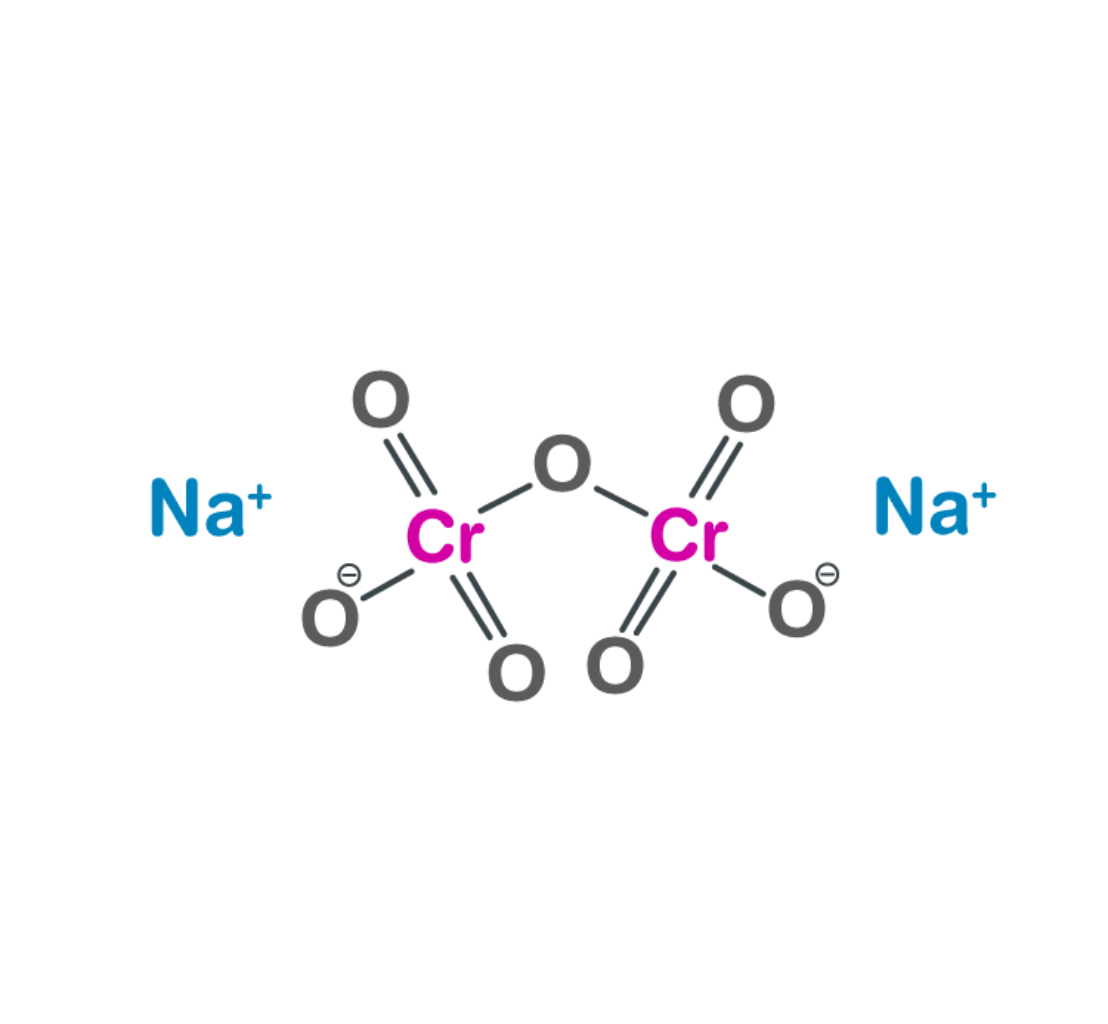
Sodium Dichromate is an orange to red colored, crystalline, inorganic compound that emits toxic chromium fumes upon heating. Sodium dichromate is highly corrosive and is a strong oxidizing agent. This substance is mainly used to produce other chromium compounds, but is also used in drilling muds, in metal treatments, in wood preservatives, in the production of dyes and organic chemicals and as a corrosion inhibitor.
A powerful oxidizer and a source for chromium-based derivatives is sodium dichromate. Applications for it include pigments and coatings, screen printing in photographic engraving, electroplating, pyrotechnics, and explosives. It is utilized in metal passivation.
Sodium dichromate functions as a corrosion inhibitor in metal treatment and as a wood preservative, shielding wood from termites and fungi. In pigment preparations like lead chromate and chrome oxide green, it is also utilized.
Apperarance

| S.No. | Description | Specifications |
|---|---|---|
| 1 | Apperarance | Bright Orange Crystals |
| 2 | Chemical formula | Na Cr2 O7 2H2 O |
| 3 | Molecular Weight | 298.0 |
| 4 | Solubility | Readily soluble in water |
| 5 | Specific Gravity at 20C | 2.35 |
| 6 | Heat of Solution cal /gm | Minus 28.2 |
| 7 | Stability in Air | Deliquescent |
Chemical Properties
| S.No. | Description | Units | Typical Values |
|---|---|---|---|
| 1 | Sodium Bichromate as Na Cr2 O7 2H2 O (%) | % w/w | 99.50 ± 0.1 |
| 2 | pH of (10% Solution) | 3.8 – 4.2 | |
| 3 | Matter insoluble in water | % w/w | 0.02 – 0.05 |
| 4 | Sulphate as SO4 (%) | % w/w | 0.10 – 0.17 |
| 5 | Chlorides as Cl (%) | % w/w | 0.10 Max |
| 6 | lron as Fe (%) | % w/w | 0.001 to 0.002 |
| 7 | Moisture at 105 ° C | % w/w | 11.00 – 11.50 |
Applications

Furthermore, sodium dichromate is also extensively utilized in the manufacturing of photovoltaic cells and solar panels, which is estimated to create opportunities for global market growth. Sodium dichromate (Na2Cr2O7) is a potent oxidizing agent commonly used in various industrial applications.
Packaging

- 25 kg and 50 kg HDPE bags with inner polyethylene layer.
- 1000 kg Jumbo bags.
Storage Condition
- Store in cool and dry places.
- Keep away from sunlight and heat sources, combustible organic materials, volatile organic compounds, flammable solvents and other readily oxidizable material
- Avoid storage on wood floors
- Remove and dispose of any spilled dichromate, don’t return to original container
- Protect against physical damage by handling vehicles like forklift or crane hooks, so as to prevent damage to bags
